INFO ON STEEL MAKING & PROCESSING
Steel is a generally hard, strong, durable and malleable alloy of iron and carbon, usually containing between 0.2% and 1.5% of carbon, often with other constituents such as manganese, chromium, nickel, molybdenum, copper, tungsten, cobalt, or silicon, depending on the desired alloy properties, and widely used as a structural material(www.dictionary.com). Depending on the carbon content of the alloy, steel is characterized as mild steel (up to 0.25%), medium carbon steel (0.25% - 0.45%), and high carbon steel (0.45% - 1.50%). When adding other constituents to iron, a wide range of alloy steels can be produced such as stainless steel and high speed steel. The process of steel-making requires first the production of cole, iron ore, and limestone; a heat source used to melt iron ore is produced. Then iron ore is melted in a furnace to produce molten iron, which is then, processed accordingly to produce steel.
1. Coke-making:
Coke and coke by-products are produced by the pyrolysis1 (heating in the absence of air) of suitable grades of coal. The process also includes the processing of coke oven gas to remove tar, ammonia (usually recovered as ammonium sulfate), phenol, naphthalene, light oil, and sulfur before the gas is used as fuel for heating the ovens. In the coke-making process, bituminous coal is fed (usually after processing operations to control the size and quality of the feed) into a series of ovens, which are sealed and heated at high temperatures in the absence of oxygen, typically in cycles lasting 14 to 36 hours. Volatile compounds that are driven off the coal are collected and processed to recover combustible gases and other by-products. The solid carbon remaining in the oven is coke. It is taken to the quench tower, where it is cooled with a water spray or by circulating an inert gas (nitrogen), a process known as dry quenching. The coke is screened and sent to a blast furnace or to storage. Thus, cole turns into a solid carbon fuel and carbon source, called coke, used to melt and reduce iron ore.
1. "Pyrolysis is the chemical decomposition of a substance by strong heating in the absence of oxygen or any other reagents. Pyrolysis is a special case of thermolysis, and is most commonly used for organic materials; extreme pyrolysis, which leaves only carbon as the residue, is called carbonization."
2. Iron-making:
During iron-making, iron ore, coke, heated air and limestone or other fluxes are fed into a blast furnace. The heated air causes the coke combustion, which provides the heat and carbon sources for iron production. Limestone or other fluxes may be added to react with and remove the acidic impurities, called slag, from the molten iron. The limestone-impurities mixtures float to the top of the molten iron and are skimmed off, after melting is complete.
Production of Molten Steel
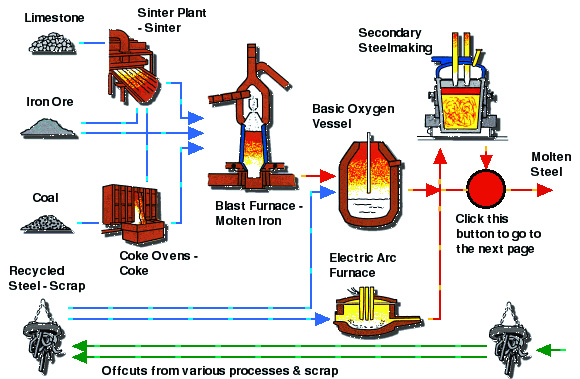
In an integrated iron and steel mill, ores are agglomerated into pellets, nodules, sinter, or briquettes for further processing. The main component of iron making worldwide is the blast furnace. Agglomerated ore is charged with coke and crushed limestone to provide both the intense heat and chemical reduction necessary to produce molten iron. The coke ovens and the blast furnaces produce combustible gases (coke oven (rich) gas and blast furnace (lean) gas) that are used throughout the mill in various heating processes. The necessary charge to produce molten pig iron consists of iron-bearing materials, coke, and flux. The charge is introduced into the furnace at the top. Blasts of heated air from large blast stoves, and in most cases gaseous, liquid, or powdered fuel, are injected into the furnace through openings (tuyeres) at the bottom of the shaft just above the hearth crucible. As the hot air encounters the coke, the coke is burned along with the injected fuels, producing the necessary heat and reducing gas to remove oxygen from the ore in the reduction process. As the iron melts, it descends and accumulates in the crucible. The molten pig iron and slag are drained from the crucible through different tapping holes. The gas that exits from the top of the furnace goes through a cleaning process. The cleaned hot gas is then used in other operations of the plant, e.g. to pre-heat the blast air, while the collected dust is sent to the sintering plant for recycling back into the blast furnace. Once fired-up, a blast furnace burns continuously until the lining needs replacement (approximately 5-6 years).
3. Steel-making:
Molten iron from the blast furnace is sent to a basic oxide furnace, which is used for the final refinement of the iron into steel. High purity oxygen is blown into the furnace and combusts carbon and silicon in the molten iron. The basic oxide furnace is fed with fluxes to remove any final impurities. Alloy materials may be added to enhance the characteristics of the steel.
Steelmaking further refines the iron by removal of carbon, silicon, sulfur, phosphorous, and manganese. In an integrated mill, the molten iron along with a certain amount of scrap is charged into a basic oxygen furnace (BOF) in which oxygen and fluxes are used to oxidize the impurities out of the iron melt. After the steel has been refined, the furnace is tilted (opposite to the charging side) and molten steel is poured out into a preheated ladle. Alloys are added to the ladle during the pour to give the steel the precise composition desired. In some steelmaking applications, further refining is conducted in the ladle to remove oxygen and sulfur from the molten steel. This is a process called "Tapping."
Another method of steel-production is through scrap recycling rather than using iron-ore. Electric arc furnaces are used for melting and refining of steel scrap, primarily in mini-mills. Electric furnaces can also use direct reduced iron as part of the charge to better control alloy content. Ladle metallurgy is often used before or after basic processing to perform specific purification steps that allow faster processing in the basic steel making operation. The electric are furnace (EAF) is the principle furnace type for the electric production of steel. The primary application of the EAF is for the re-melting of steel scrap; however, EAFs can be charged with limited amounts of iron scrap, pig iron, and direct reduced iron.
After tapping from a BOP or EAF, molten steel for high quality or specialty applications is subjected to further refining in a number of alternative processes collectively known as ladle metallurgy, ladle refining, or secondary steelmaking. The objectives of these processes are: Deoxidization - Removal of Oxygen, Degassing - Removal of Hydrogen, Desulphurization - To sulfur concentrations as low as 0.002%, Micro-cleanliness - Removal of undesirable nonmetallic elements, Inclusion morphology - Changing the composition of remaining impurities to improve the, microstructure of the steel, Mechanical properties - Increases toughness, ductility, and transverse properties.
In continuous casting, the molten steel from the steelmaking operation or ladle metallurgy step is cast directly into semi-finished shapes (slabs, blooms, and billets). Continuous casting represents a tremendous savings in time, labor, energy, and capital. By casting the steel directly into semi-finished shapes, the following steps are eliminated: ingot teeming, stripping, and transfer; soaking pits; and primary rolling. Continuous casting also increases yield and product quality.
Thin slab casting is the centerpiece of a new technology that could revolutionize the competitive structure of steelmaking both in the U.S. and worldwide by making flat rolling accessible to mini-mills. Unlike conventional casting that produces a slab with up to a 10" section; thin slab casters produce a slab from 2"-3.5" thick that is integrated with a strip mill. The technology eliminates the large roughing mills required to work the thick slabs, and integrates slab production and sheet and strip rolling, greatly reducing reheating requirements.
Otherwise, in the ingot casting process, refined molten steel is tapped into refractory lined ladles. The steel is then teemed (poured) into a series of ingot molds. After the ingots solidify, the ingot molds are stripped and the ingots are placed in soaking pits for heating and to equalize the internal and external temperature. Following the soak, the ingots are hot rolled in large primary rolling mills to produce slabs, blooms, or billets depending on the final products desired.
Finally, slabs, blooms, or billets are taken through a secondary finishing process to convert such semi-finished shapes into final products offered for sale.
i. Plate, Sheet, and Strip Mills
Nearly 60% of steel products come from plate, sheet, and strip mills. All processes begin with slab reheating to rolling temperature - with an increasing share being hot charged as part of a thin slab casting/direct rolling process. Plate mills generally have one roughing stand and one finishing stand (both reversing mills) with a final leveler. Sheet mills generally have continuous roughing and finishing stands that reduce the section of the slab to about 1/10". The sheet is cooled and coiled and then fed to pickling tanks to remove surface oxidation. Some sheet is fed to cold reduction mills that reduce the 1/10" hot rolled sheet to 0.03" sheet. The cold rolled coils are annealed and then passed to a final temper mill. Many sheet products are coated, the description of these coating processes is provided in the next process section.
ii. Welded Pipe and Tubing
Coils of skelp (steel sheet or plate from which welded pipe and tubing is made) from a sheet rolling process are uncoiled and welded together to form a continuous feed. The skelp makes several loops through a continuous reheating furnace before it is formed into pipe and seam welded. The pipe is then further rolled on a sizing mill before cutting, cooling, testing, and shipping.
iii. Structural and Bar Mills
In structural mills, steel blooms or billets are brought to uniform temperature in continuous reheat furnaces and then passed through roughing, intermediate, and finishing mills to produce the desired shapes: I Beam, channel, angle, zee. Bar mills start with continuous billet reheaters followed by roughing and finishing stands. Some product is coiled and the other is finished and cut into straight lengths.
iv. Rod and Wire Mills
Rod mills are similar to bar mills having continuous billet reheating, roughing, and intermediate stands. The rod mill has multiple finishing sections where rods down to 1/4" are coiled at the rate of 8,500 feet per minute. In wire mills, coils of rod are cleaned, coated with a lubricant and baked, and then drawn on a continuous wire drawing frame. Wire can also be annealed and coated depending on the intended use.
v. Seamless and Specialty Tubing
Cut billet rounds are heated, often in a rotary hearth and then pierced by a center die, followed by hot extruding to the desired pipe diameter and wall thickness. These pipe sections can also be cold worked by cold drawing or tube reducing.
vi. Specialty Products
Stainless steel, tool steels, and large forgings have their own unique process steps. These processes are more likely to use batch furnaces due to small volume and changing shape requirements.
References:
1) Metals Processing Advisor, "Iron and Steel Overview: An in depth look at the
Iron and Steel Industries and associated process
equipment."
2) World-Bank. "Industrial Pollution Prevention and Abatement:
Coke Manufacturing." Washington, D.C. July 1998.







